Indications
o Acute neck mass/swelling
o Cervical lymphadenopathy
o Suspected neck skin/soft tissue infection
Equipment
o Ultrasound machine
o High-Frequency Linear transducer (7-12 MHz)
o Gel for acoustic interface
o Acute neck mass/swelling
o Cervical lymphadenopathy
o Suspected neck skin/soft tissue infection
o Ultrasound machine
o High-Frequency Linear transducer (7-12 MHz)
o Gel for acoustic interface
Neck mass is a common presenting complaint in the pediatric population with a broad differential diagnosis. Point of care ultrasound (PoCUS) provides a rapid, non-invasive, and real-time evaluation of these masses. Indications for bedside ultrasound assessment include palpable neck masses, cervical lymphadenopathy, and evaluation of suspected neck infections or malignancies.
Common causes of neck swelling in children include enlarged lymph nodes, most often due to inflammatory or reactive responses, infections, or – less commonly – malignancies. Congenital developmental masses, benign neoplastic lesions, and salivary gland infections also contribute to the range of potential causes. Malignancies can also be a cause of neck swelling and are important to consider in the differential diagnosis although rare.
In the evaluation of a neck mass, a comprehensive approach is essential. This involves gathering a detailed medical history, conducting a thorough physical examination, and investigations including laboratory work and imaging studies when clinically indicated (1). Cervical lymphadenopathy ranks among the most common contributors to neck swelling in children. This module will specifically concentrate on the assessment of the diverse causes of lymph node swelling using PoCUS.
Ultrasound (US) is considered the primary imaging modality of choice for evaluating inflammatory neck masses because of its widespread availability, excellent resolution, and lack of ionizing radiation (2,3). Ultrasonography can define the size, shape, and characteristics of the mass and any associated vascularity. Ultrasonography can also define the tissues surrounding the mass, providing more clues to the etiology. Further, US is safe, cost-effective, and available in real time. It is also readily available in most hospital settings.
PoCUS imaging of neck masses compares favourably to formal radiological assessments. One study assessed PoCUS with radiology department ultrasonography (RaDUS), while another compared PoCUS with multiple radiological evaluations (RaDUS, CT, or both). Both studies involved pediatric patients aged 0 to 18 years presenting with neck swelling to the emergency department. POCUS trained pediatric emergency physicians concurred with radiology-based imaging diagnoses in 77% -78% of cases in two studies with. Corresponding kappa values were also comparable—0.69 (95% CI 0.44–0.94) and 0.71 (95% CI 0.60-0.83) respectively (4,5).
These studies not only highlight the diagnostic accuracy of PoCUS but also shed light on its ability to identify a wide range of neck pathologies. The spectrum of diagnoses includes, but is not limited to, lymph node abnormalities (infections, inflammation, and malignancies), solid tumors, congenital cysts, parotitis, and others. These findings support PoCUS as a reliable, rapid, and non-invasive diagnostic tool in the pediatric emergency setting, offering a valuable complement to history, physical exam, and other imaging modalities.
In addition, performing PoCUS was found to reduce emergency department length of stay i in children presenting with neck masses (5,6).

**To continue through to the course, make sure to select the “Mark as Completed” button below, and at the end of each lesson page that follows.
**To unlock access to the first quiz, make sure to select the “Mark as Completed” button below
The sensitivity for PoCUS in diagnosing appendicitis in pediatrics ranges from 89-96% [8,9], depending on operator experience, age, patient body habitus, and appendix position. The most significant pitfall is that the normal appendix is far more difficult to identify than the abnormal appendix, leading to a higher rate of nondiagnostic studies. Additionally, retrocecal or pelvic positioning (Figure 15), perforation, overlying bowel gas (figure 16), and increased body habitus can obscure visualization, making diagnosis more challenging.
A common pitfall is mistaking normal small bowel for the appendix; to avoid this, confirm that the structure is blind-ended and check for peristalsis (as active movement suggests bowel rather than the appendix) and assess size and compressibility (via graded compressions), as small bowel is typically larger in diameter and more compressible than the appendix.
Other limitations include patient discomfort and pain during the examination. This can make the procedure more challenging, particularly in children with more severe symptoms, as they may struggle to remain still or tolerate the pressure applied during the examination. To optimize imaging, consider using analgesia, ensuring parental presence, and utilizing distraction techniques to help the child remain still and tolerate the pressure applied during the examination.
Another limitation to consider is the visualization of the entire appendix. While ideally the entire appendix from the cecal junction to the tip should be visualized, this can be challenging and is not always possible in practice.
Proper image acquisition and review are essential to ensure accurate identification of the appendix, with careful correlation to clinical findings.
Figure 17: Retrocecal position of the appendix. The cecum is seen to screen left, and the terminal ileum, which looks like a cut tomato, is to screen right. Video courtesy of Dave Kirschner, used with permission.
Figure 18: Cross-section of the Appendix in the RLQ intermittently visible, obscured at times by overlying bowel gas. Video courtesy of Dave Kirschner, used with permission.
Ultrasound findings in acute appendicitis typically include [16,17]:

*Most typically a total diameter of >6mm has been used, however it is important to note that there is variability in normal appendix size and some institutions have had success with adjusted criteria to increase specificity and therefore decrease the negative laparotomy rate [18]
** In cases of acute appendicitis, the appendix typically does not compress under probe pressure [19]. However, if perforation has occurred, the appendix may appear compressible, which can complicate the assessment [20]
Figure 9: Inflamed appendix long axis with fluid within the lumen and surrounding hyperechoic fat. Video courtesy of Dave Kirschner, used with permission
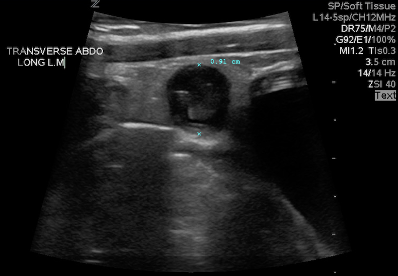
Figure 10: Inflamed appendix in short axis. Image courtesy of Dave Kirschner, used with permission
Figure 11: Easily visualized round, thick-walled structure – Cross section of inflamed appendix. Video courtesy of Dave Kirschner, used with permission.


Figure 12: Hyperechoic, reactive fat surrounding the inflamed appendix. Borbély Márton, CC BY-SA 4.0 via Wikimedia Commons [22]
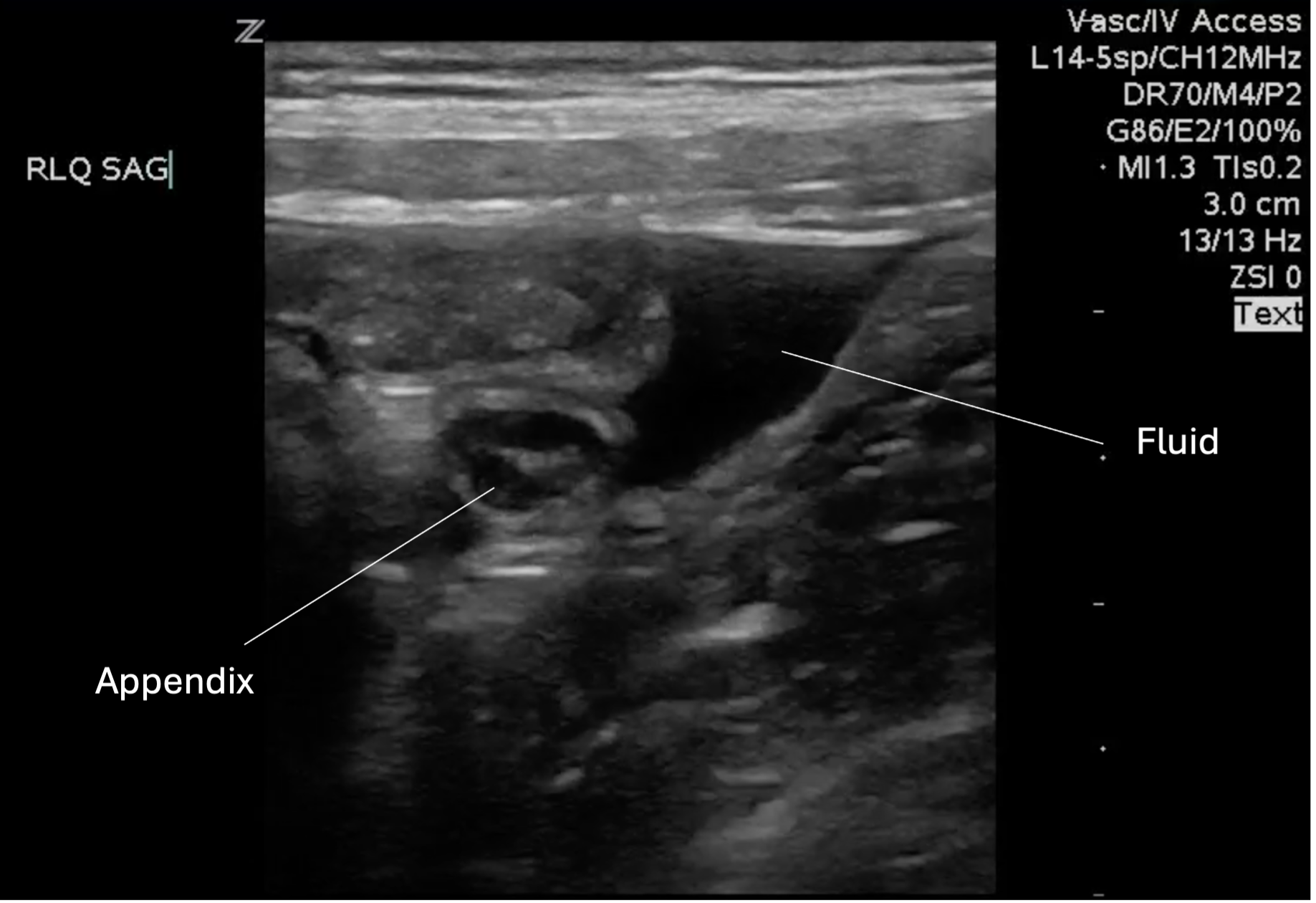
Figure 13: Peri-appendiceal simple fluid collection
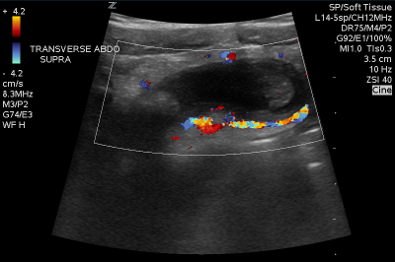
Figure 14: Inflamed appendix with color doppler displaying the “ring of fire” appearance. Image courtesy of Dave Kirschner, used with permission.
Figure 15: Inflamed appendix with appendicolith. Video courtesy of Dave Kirschner, used with permission.
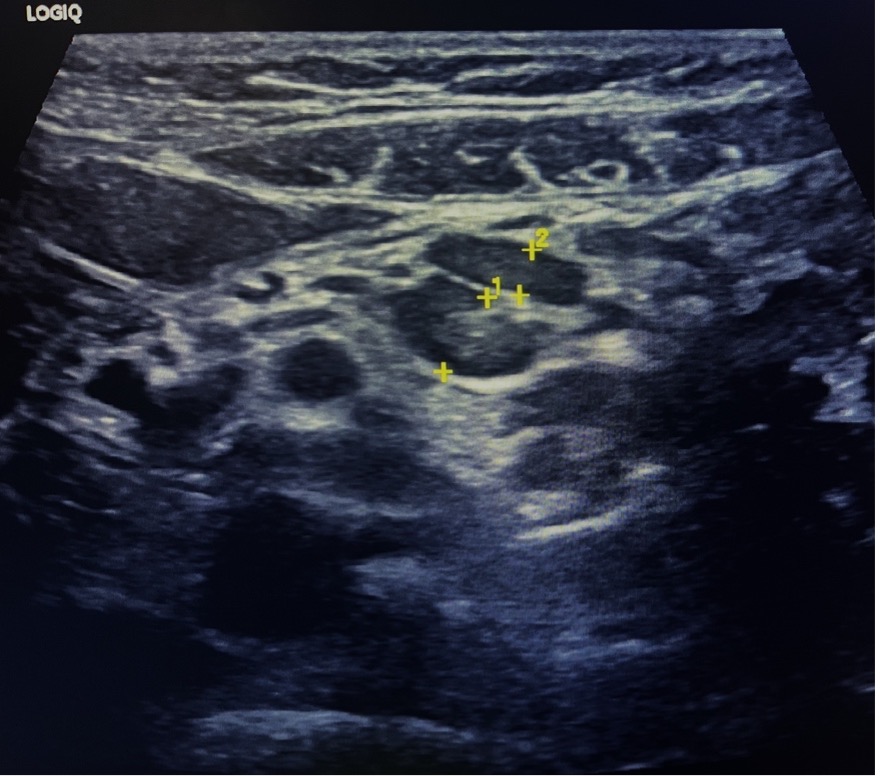
Figure 16: Localized lymphadenopathy at the RLQ
On ultrasound, the appendix appears as a blind-ended tubular structure in the long axis and a target-like or oval structure in the short axis.
| A normal appendix has the following sonographic features: | |
| – | Thin (≤ 6mm) |
| – | Layered appearance (gut signature). The lumen may contain air, fluid or debris |
| – | Compressible with gentle probe pressure |
| – | Minimal vascularity |
| – | No peristalsis |
| – | Difficult to locate (due to variable tip position) |
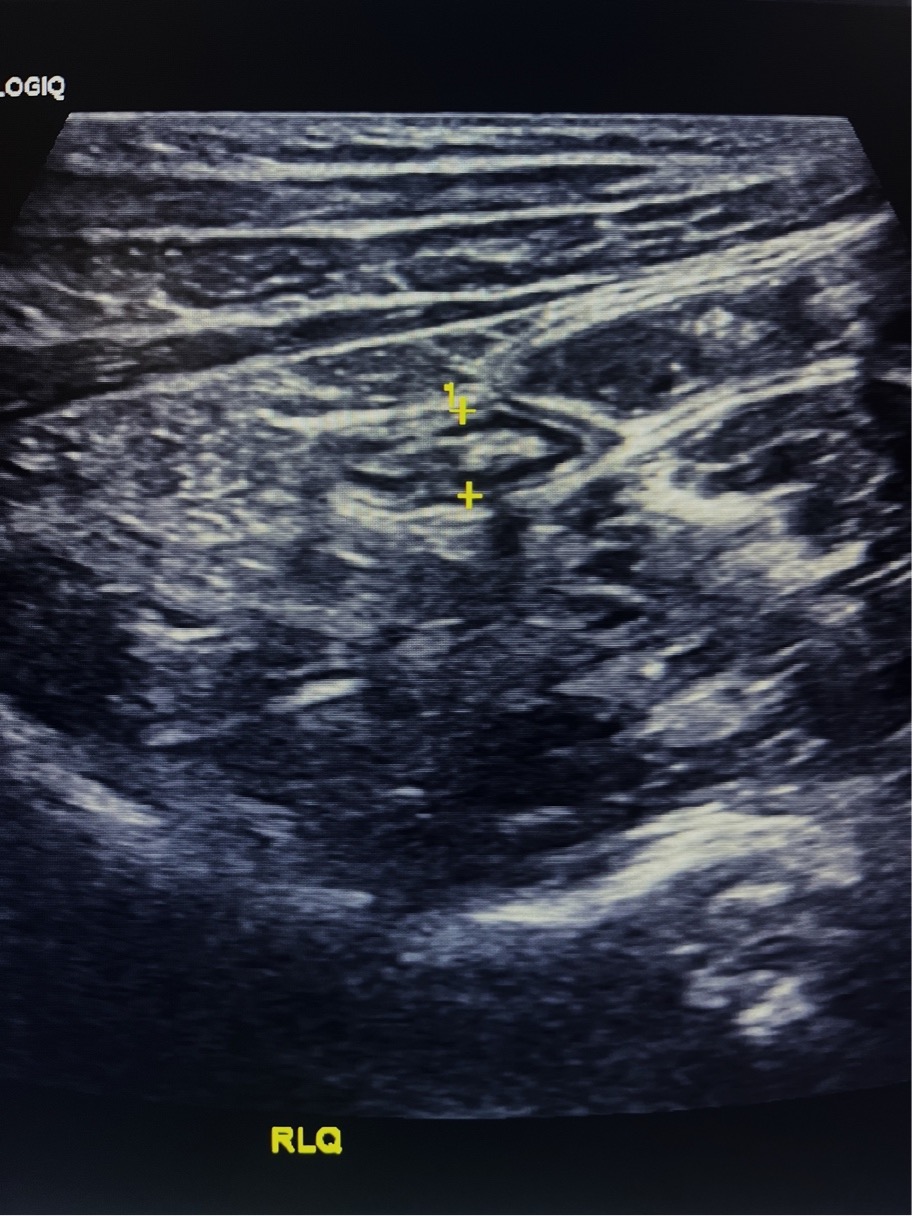
Figure 6. Long-axis view of the appendix tip, demonstrating a normal appearance
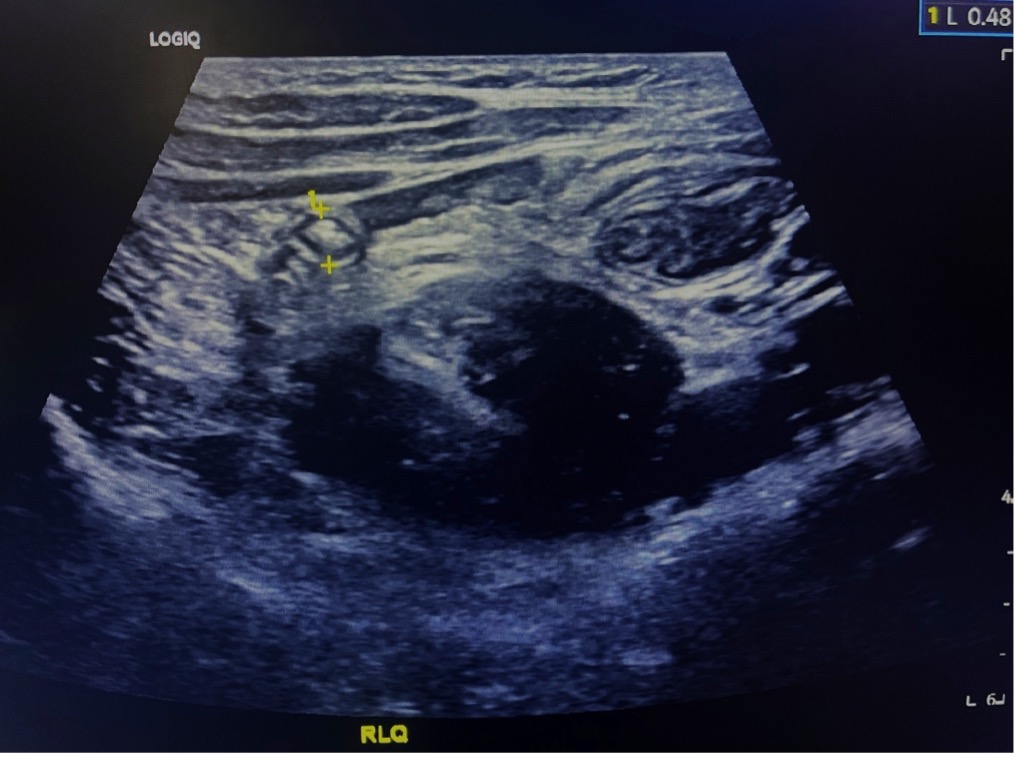
Figure 7 Normal appendix in short axis
Figure 8: Normal appendix in cross-section (yellow arrow) off the colon (pink arrow) medially. Video courtesy of Dave Kirschner, used with permission.