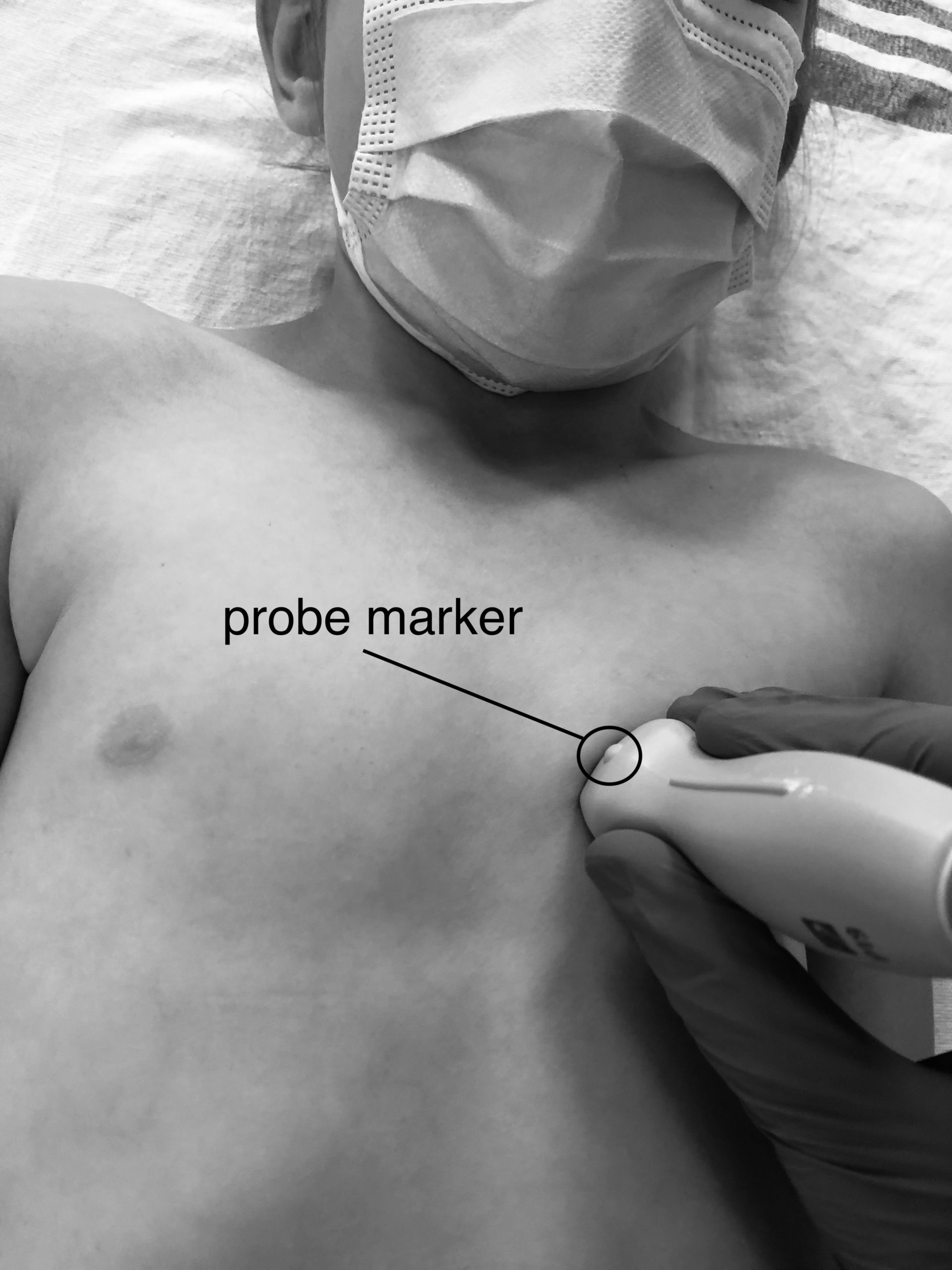
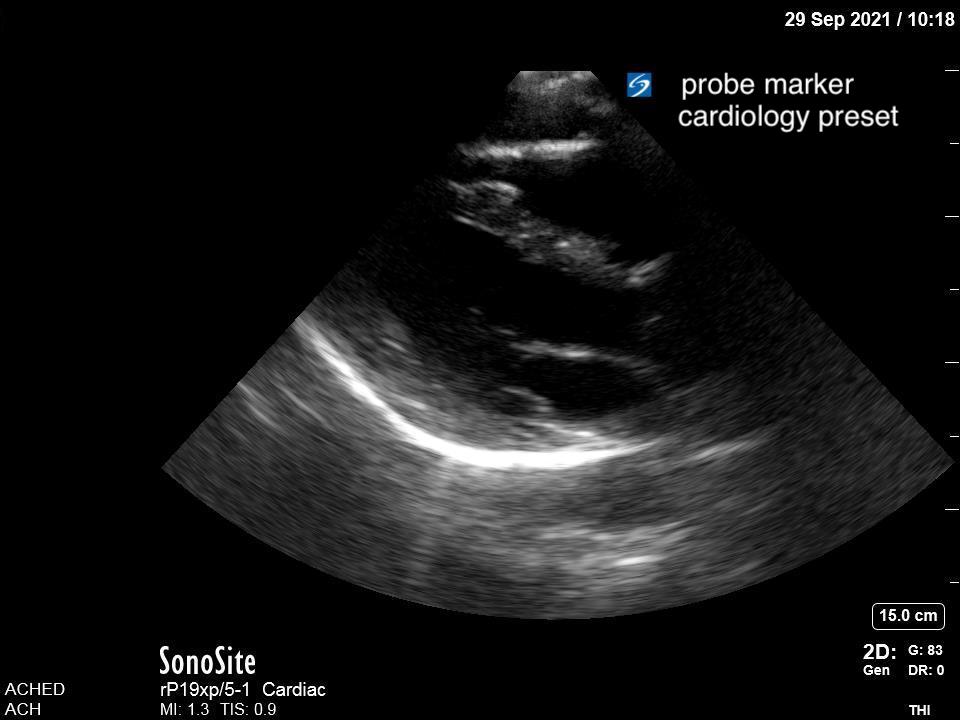
Introduction
Pneumonia is a leading cause of death in children around the world. The most common initial test for pneumonia is chest radiograph/x-ray (CXR) despite it having limited sensitivity and exposing children to radiation. Growing evidence shows that point-of-care ultrasound (PoCUS) can reliably detect lung consolidation with equal, if not better, sensitivity than CXR. Computed tomography (CT) provides the best test characteristics but is impractical and carries the cost of significant radiation.
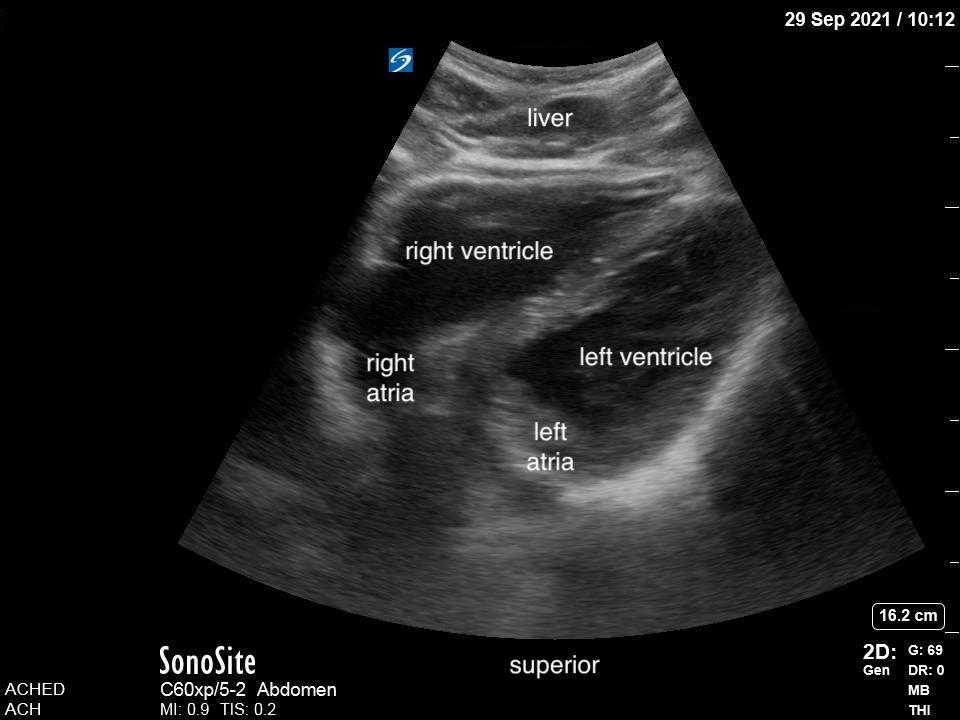
Lung ultrasound (LUS) has excellent test characteristics, is noninvasive, delivers no ionizing radiation, and does not require a patient to be moved to a radiology department. For these reasons there is great potential for its use at the bedside in the diagnosis of pneumonia in children.
Why Ultrasound?
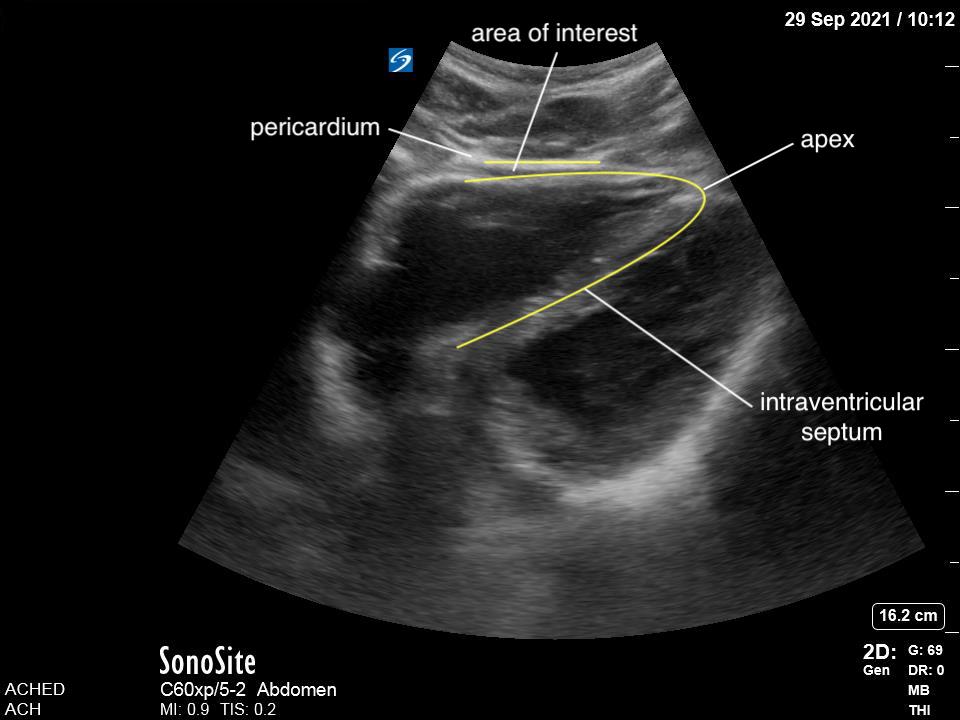
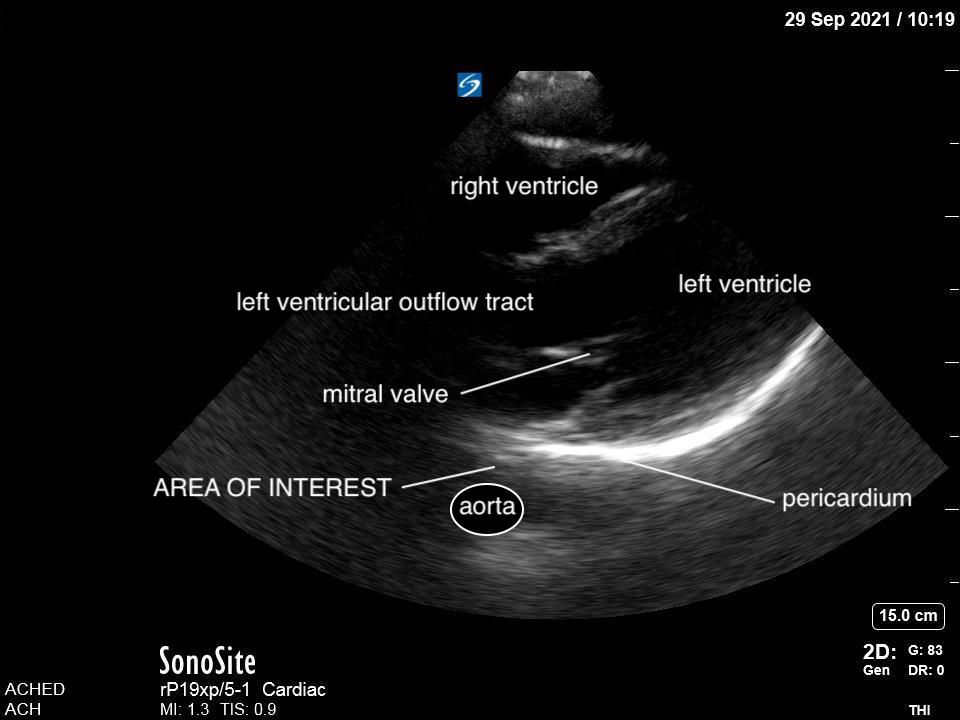
Given that air scatters ultrasound waves, it was traditionally thought that lung ultrasound would not be useful to detect pathology. However, over the last two decades there is a growing body of evidence supporting the use of ultrasound in various lung pathologies. Since lung pathology, such as pneumonia, leads to edema and fluid accumulation within the alveoli, areas of consolidation can be seen on ultrasound as long as this fluid reaches the pleural line. Fortunately, this is the case in most patients, particularly in children who have small lungs.
Several meta-analyses examining test characteristics of LUS for pediatric pneumonia have been published (1,2,3). The initial meta-analysis published in 2015 found a pooled sensitivity of 96% and specificity of 93% and an area under the ROC of 0.98 (3). All studies revealed LUS as equal if not superior to detecting consolidation compared to CXR (1,2,3). In the adult literature, a study comparing the test characteristics of LUS and CXR to the gold standard CT revealed a significantly better sensitivity of lung ultrasound: 86% vs 64%, with similar specificities. (4)
Jones et al. published a randomized-control trial examining the feasibility of replacing CXR with LUS for the diagnosis of pneumonia in children. They showed a 30-60% reduction in the use of CXR, depending on the level of experience of the ultrasonographer, as well as decreased ED length of stay. The inter-rater reliability for LUS in this practical study was 0.81, showing excellent agreement (5). A meta-analysis examining accuracy of LUS in novice vs advanced sonographers did find that novice sonographers had decreased accuracy, but the sensitivity and specificity remained 80% and 96% respectively, with an area under the ROC of 0.97 (6). Though, the question does remain as to what makes someone novice vs expert in LUS.
Additional questions about LUS remain to be answered. Specifically, what is the value of each abnormal finding on LUS and which findings are indicative of bacterial pneumonia necessitating antibiotic treatment? Studies agree that findings such as hepatization, air bronchograms and large subpleural consolidations are consistent with bacterial pneumonia, but focal B-lines, small subpleural consolidation and irregularities of the pleural lining can also be found in viral etiologies. A recent study looking at the significance of sub-centimeter, subpleural consolidations concluded that as an isolated finding, these are not indicative of bacterial pneumonia (7). Further study in this area continues to emerge. Jones et al. raised caution that LUS could unintentionally increase antibiotic usage rates if there is no clear definition of bacterial pneumonia (5).
| Modality | Sensitivity | Specificity | Area under the ROC |
| LUS | 95.5% (93.6 – 97.1) | 95.3% (91.1 – 98.3%) | 0.98 |
| CXR | 86.8% (83.3 – 90.0) | 98.2% (95.7 – 99.6) |