Pitfalls
Subxiphoid View
False Positive – Epicardial Fat Pad
-
- An epicardial fat pad can be found in young healthy individuals.
- It will move synchronously with the heart and will not encompass the heart.
- The epicardial fat pad will also be more prominent anteriorly, while PCE will be more prominent posteriorly.
FIGURE 13: Epicardial fat pad on subxiphoid view.
Parasternal Long View
False Positive – Pleural Effusion
-
- Pleural effusions can be seen on the parasternal long view but will typically be found in the far field, deep to the descending aorta (reminder: a pericardial effusion will be seen near field to the descending aorta).
What is NOT normal?
Subxiphoid View
A pericardial effusion will first be visible in the posterior part of the pericardium given the patient is laying supine. The fluid will be seen between the myocardium and the liver. As fluid accumulates, the effusion will progress to encompass more of the heart. Effusion will be seen as an anechoic collection surround the heart, as if the heart is beating within a sac of fluid.
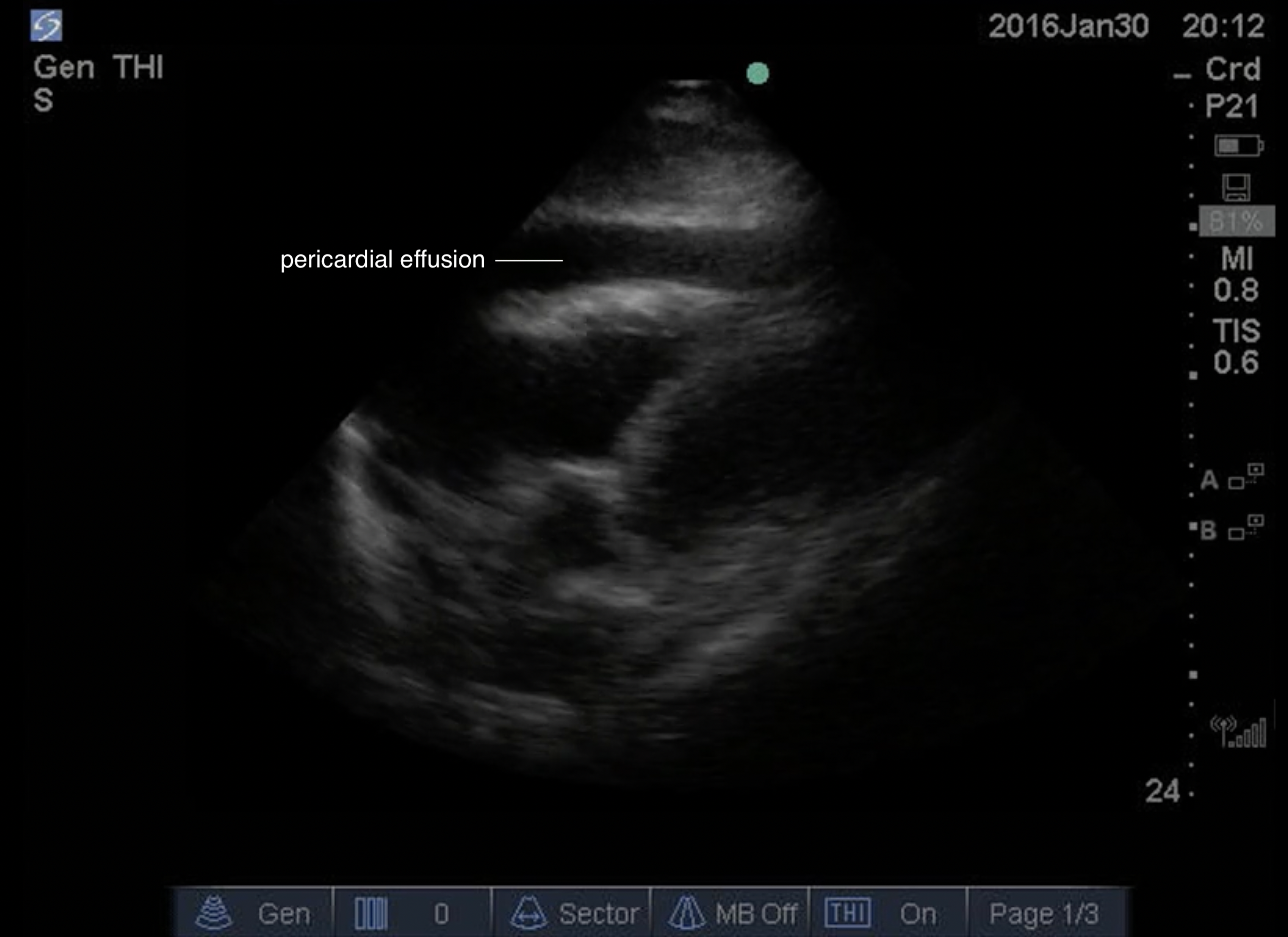
FIGURE 10: Pericardial Effusion Subxiphoid View
Parasternal Long View:
A pericardial effusion will be seen between the left atrium and ventricle and the descending aorta.
It will again appear as an anechoic, black, collection on your screen.
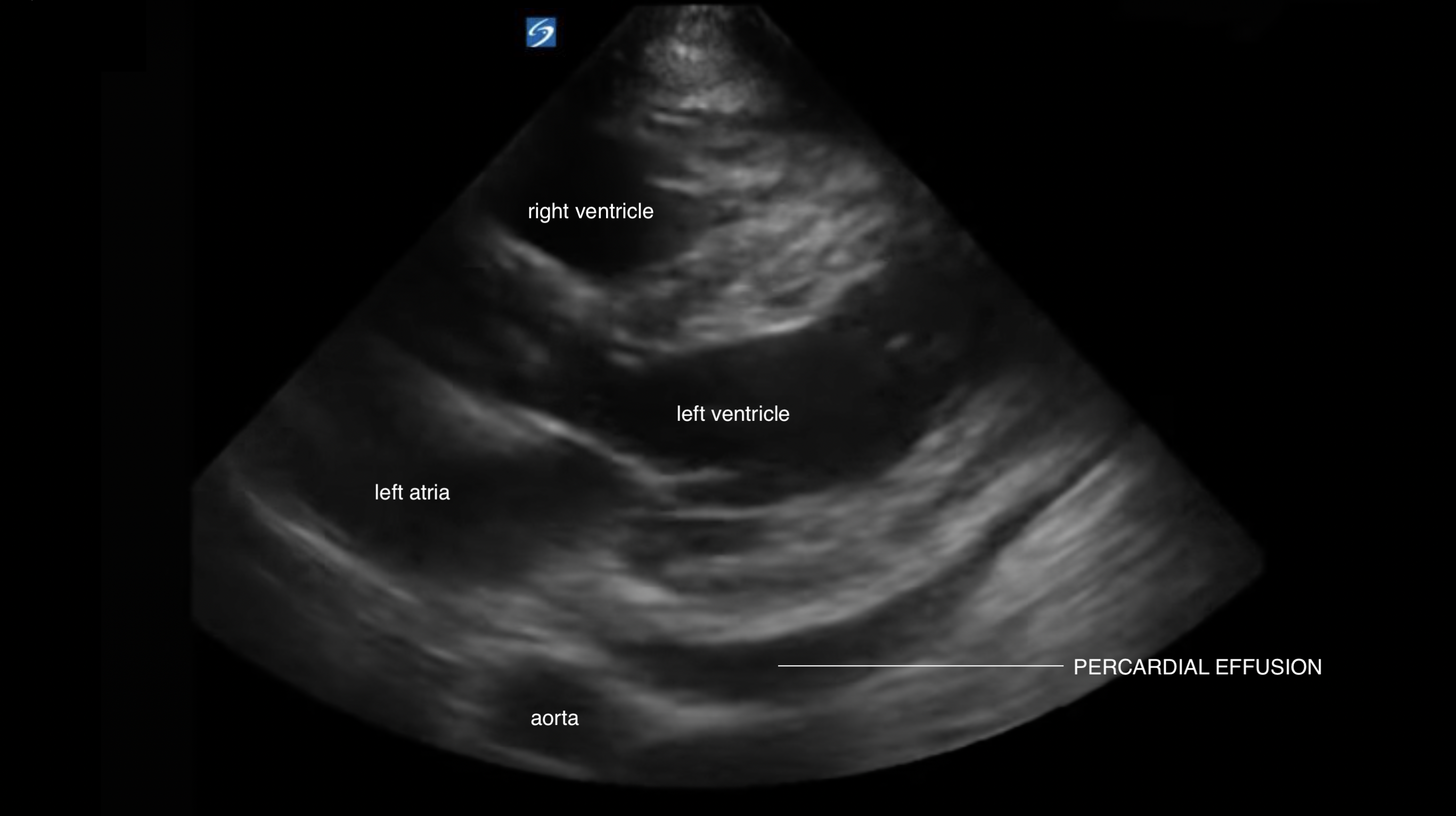
FIGURE 11: Pericardial Effusion Parasternal Long View
How to Distinguish Between Pleural Effusion and Pericardial Effusion
Pericardial Effusion
- Crosses the midline
- Separates descending aorta from pericardium
- Beware tamponade physiology
Pleural Effusion
- Accumulates posterolateral to the descending aorta
- Typically, not present in the subxiphoid view given no pleural reflection between the liver & heart

FIGURE 12: Pericardial effusion vs pleural effusion on parasternal long view [8].
Tamponade
Cardiac Tamponade is a clinical diagnosis. Hemodynamic instability within the context of pericardial effusion should be taken seriously and prompt consideration of tamponade. While there are sonographic clues to tamponade these are beyond the scope of this module and will be discussed in a future ”advanced“ module
What is normal?
Our area of interest is the space between the pericardium and the myocardium of the left ventricle, extending to the apex of the heart. It is normal for some patients to have visible trace pericardial fluid, which will act as a lubricant for cardiac motion. This, however, will be minimal.
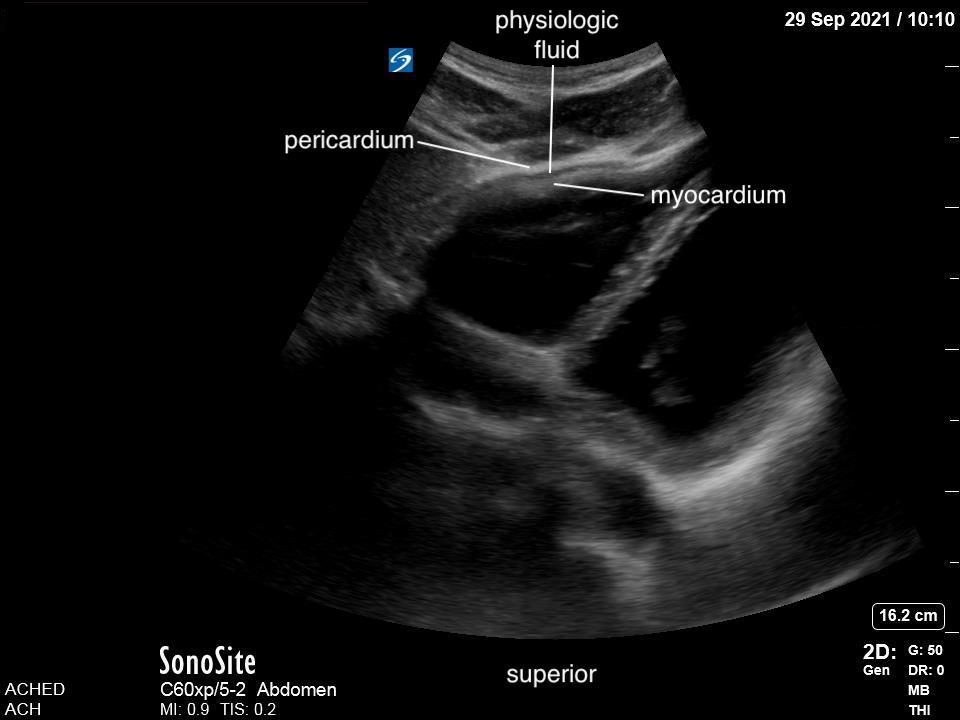
FIGURE 8: Subxiphoid. Physiologic free fluid.
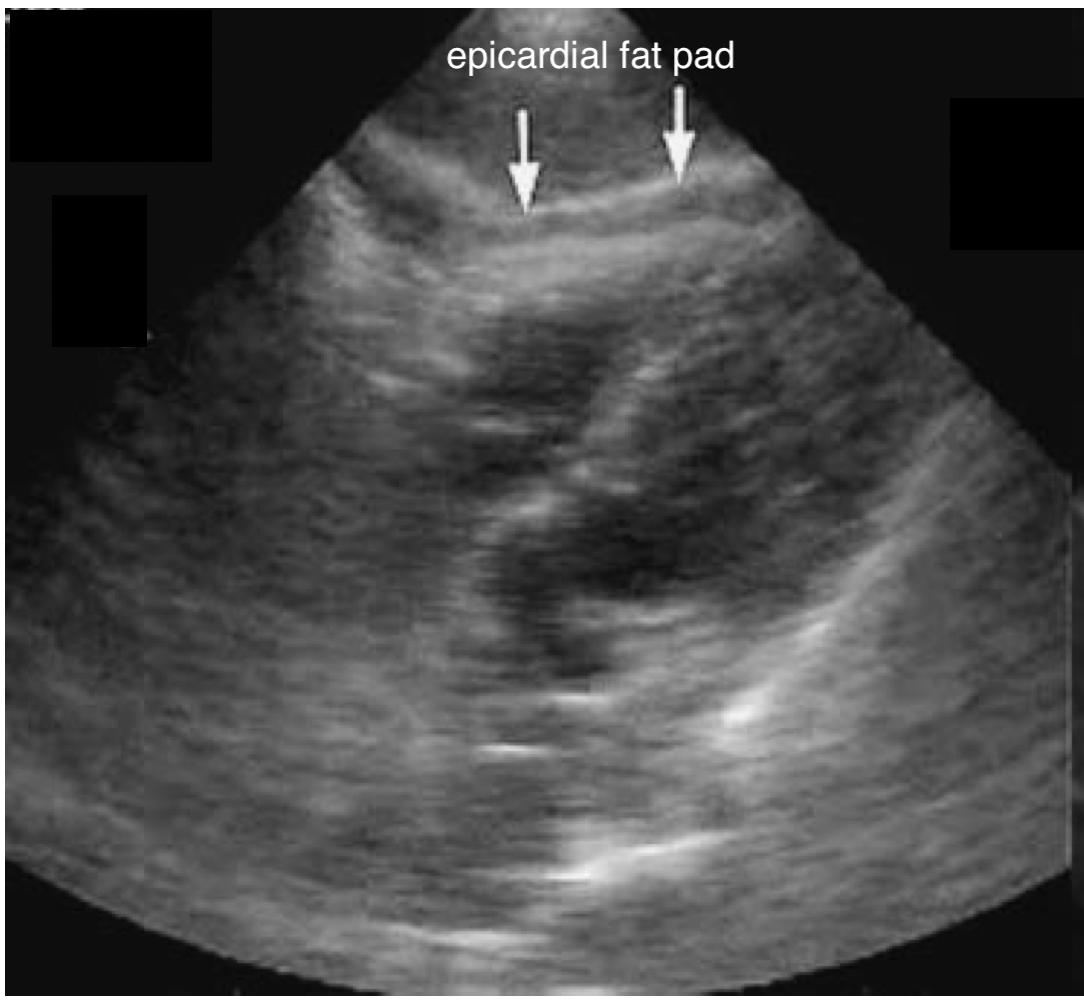
FIGURE 9: Subxiphoid. Pericardial fat pad.
It is also normal for some patients to have a pericardial fat pad. This fat pad sits between the pericardium and the myocardium and has a similar, black, appearance. Unlike fluid, however, the fat pad will be more obvious anteriorly, and disappear as you move posteriorly. Unlike fluid which will move posteriorly to the most dependent area. Also, the fat pads will have very little variation with contraction, while pericardial effusions will show significant variation with contraction.
Pitfalls
Disease states other than pneumonia can also result in appearance of consolidation on ultrasound—just as in X-ray. Consolidation is not specific to pneumonia and must be clinically correlated. Any disease state that results in loss of aeration of alveoli either through fluid accumulation or collapse, including pulmonary contusions, hemorrhage, and atelectasis, can appear as areas of sonographic consolidation.
While there are some findings that can help differentiate alveolar disease from atelectasis, these are not always reliable. In pneumonia, gas moving within the bronchioles of consolidated lung can sometimes be seen and will appear as bright dots moving in a liner fashion through an area of consolidation. These are known as dynamic air bronchograms and are generally thought to rule out atelectasis. Atelectasis can also be differentiated from pneumonia in that surrounding b-lines are unlikely to be present. Color doppler can be applied to the area of hepatization to assess flow to the area, which will be decreased or absent in cases of atelectasis but normal or increased in pneumonia.
Video 2: Dynamic air bronchogram differentiating consolidation from atelectasis.
In children the thymus can sometimes be visualized and confused for hepatized lung. The thymus appears as hypoechoic structure with bright echogenic foci scattered throughout and is often described as a “stary sky” appearance. Apart from its anatomic location on the chest, the thymus also has clearly demarcated margins due to a fibrous capsule and no surrounding b-lines or shred sign. Additionally, if color doppler is applied there is hypo vascularity compared to an area of consolidated lung.
Video 3: Thymus, well demarcated hypodense area with a “starry sky” appearance.
If the probe is not directly over the lung but is over the liver or spleen, the diaphragm can at times act as a reflective surface giving the mirror image of the liver or spleen appearing above the diaphragm. This should not be mistaken with hepatized lung—hepatized lung can be imaged directly through the pleura and will not disappear with fanning of the probe. A mirror image will disappear as the transducer is fanned or moved cranially above the diaphragm.
Ultimately clinical context is most useful in determining the etiology of positive ultrasound findings.
Also remember:
- Some areas of lung are difficult to access and image (supraclavicular, scapular, axillary)
- Consolidation that does not reach the pleural line cannot be seen with LUS
- Early pneumonia without consolidation will mimic other aetiologies
What is NOT normal?
As pneumonia develops edema begins to collect in the lung, allowing for visualization of lung on ultrasound. B-lines represent thickening of the lung parenchyma—most often secondary to edema and appear as sharp, vertical lines arising from the pleura and extending to the base of the image on the screen. B-lines obscure A-lines and move in concert with lung sliding (figure 4). 1-2 B-lines per field of view can be normal in dependent regions or areas of interlobar fissures and should be clinically correlated. Localized B-lines may be the only finding early in disease course and they can often be found adjacent to areas of consolidation. While focal B-lines may indicate a bacterial pneumonia they are non-specific and can also be seen in viral etiologies. Additionally, when B-lines are found diffusely they are more likely to indicate other disease states such as cardiogenic pulmonary edema or viral pneumonia (8). B-lines and interstitial syndromes are discussed in detail in a dedicated KidSONO module.
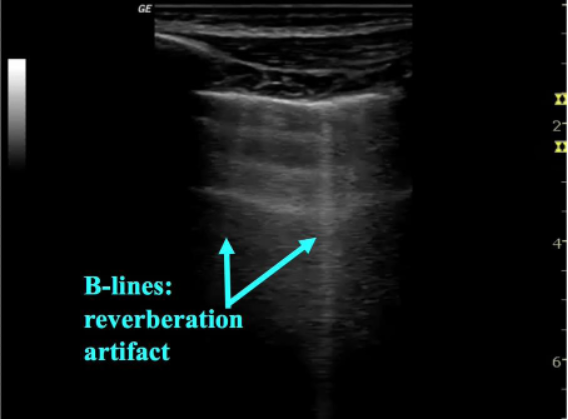
Figure 4: B-lines, indicating interstitial thickening
As pneumonia progresses and fluid starts to leak into alveoli, dark, irregular subpleural consolidations can be seen. They are backed by an irregular posterior border indicating transition from non-aerated to aerated alveoli—this is called the shred sign (figure 5). Subpleural consolidations that are greater than 1cm in diameter are generally considered to be indicative of bacterial pneumonia—smaller consolidations can also be found in viral processes such and bronchiolitis and viral pneumonias so clinical judgement is needed.
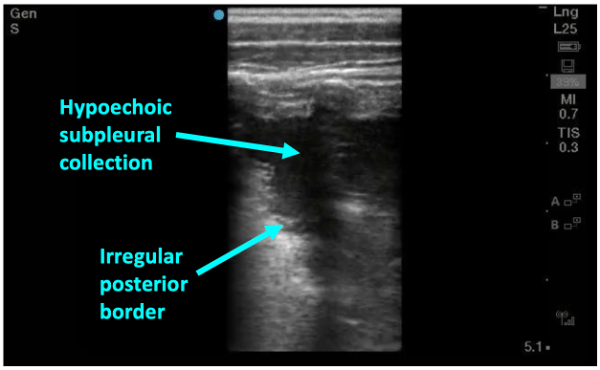
Figure 5: Sonographic consolidation with hypoechoic collection abutting the pleural line with an irregular posterior border– the “shred sign”
In more severe disease a dense area of consolidated lung begins to look like liver parenchyma on ultrasound. This is described as “hepatization” (figure 6).

Figure 6: Advanced disease with dense consolidation known as “hepatization” of the lung
Keep in mind LUS is also more sensitive than CXR for detecting pleural effusions, with just 10 mL detected on LUS, in comparison to 200mL required to be detected on CXR (9). If a parapneumonic effusion is present, LUS will easily find the anechoic area, typically at the base or more dependent areas. PoCUS for the detection of pleural effusions is covered in a dedicated module.
What is normal?
In normal lung, the pleura is easily visualized as ultrasound waves are deflected by aerated lung. Reverberation artifacts from the pleural line (secondary to the significant change in acoustic impedance at the pleural-lung interface) generate horizontally arranged artifacts called A-lines (figure 2). Other normal structures visualized are the rib and corresponding rib shadowing below, and at the base of the lung the double line of the diaphragm muscle (figure 3).
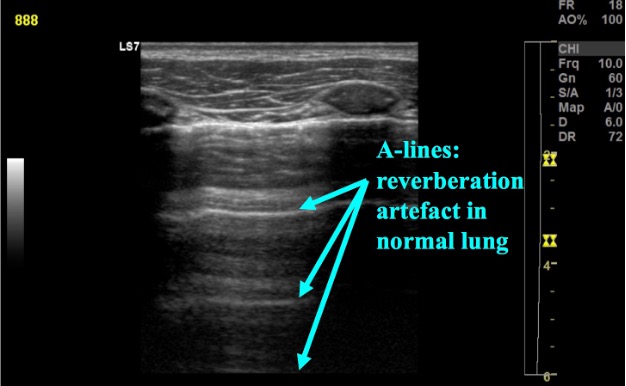
Figure 2: Normal lung with A-line reverberation artefact
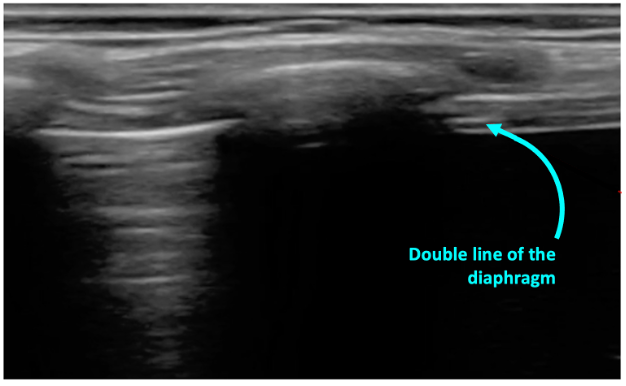
Figure 3: Normal lung base with the beginning of the diaphgram noted on screen right
What am I looking at?
It is first important to note the following normal anatomic structures and their sonographic appearance (video 1).
Chest wall:
- Most superficial structure
- Hypoechoic with irregular fascial lines
Ribs:
- Oval, hyperechoic periosteumo
- Dark shadow behind
Pleural line:
- Hyperechoic horizontal line
- Runs between and deep to the ribs
Lung:
- Deep to the pleural line
- Uniform grey haze
Video 1: Normal lung anatomy
Indications
- Dyspnea
- Cough
- Fever without a source
Equipment
- Ultrasound machine
- High frequency linear probe (6-12 MHz) is most used, although a curvilinear or phased array probe may also be used
- Gel
Technique
- Position the patient: It is easiest to completely expose the thorax. LUS can be conducted with the patient in any number of positions; in the parent’s arms (helpful for posterior exam), seated on or lying in a stretcher (lateral decubitus can be used to examine the posterior chest).
- Warm the gel if possible: Younger or sleeping patients may respond better if the gel is warmed. Consider warming the gel between your gloved hands and applying a layer of warmer gel to the chest.
- Scan the patient:
- Set the depth to between 5 – 10 cm, choosing the smallest depth that gives an appropriate image to maximize resolution.
- Hold the probe with the marker pointed towards the patient’s head. Starting most cephalad with the probe in a sagittal orientation scan from the lung apex to the diaphragm.
- Complete this process in 6 planes: posterior between the spine and the scapula left and right chest, at the mid-axillary line left and right chest, and anteriorly at the midclavicular line left and right chest (figure 1)
- For any abnormalities detected, the area should be investigated more by sliding the probe along the rib space or changing the probe to a transverse view to scan in-between the ribs.
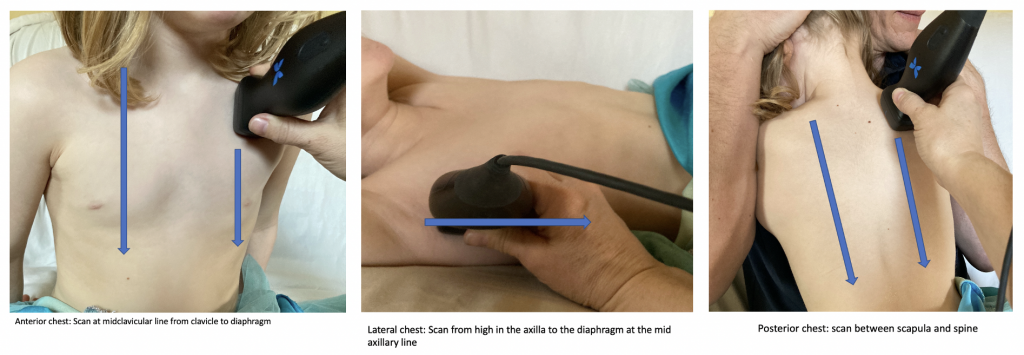
Figure 1: Scanning technique for pneumonia











