Introduction
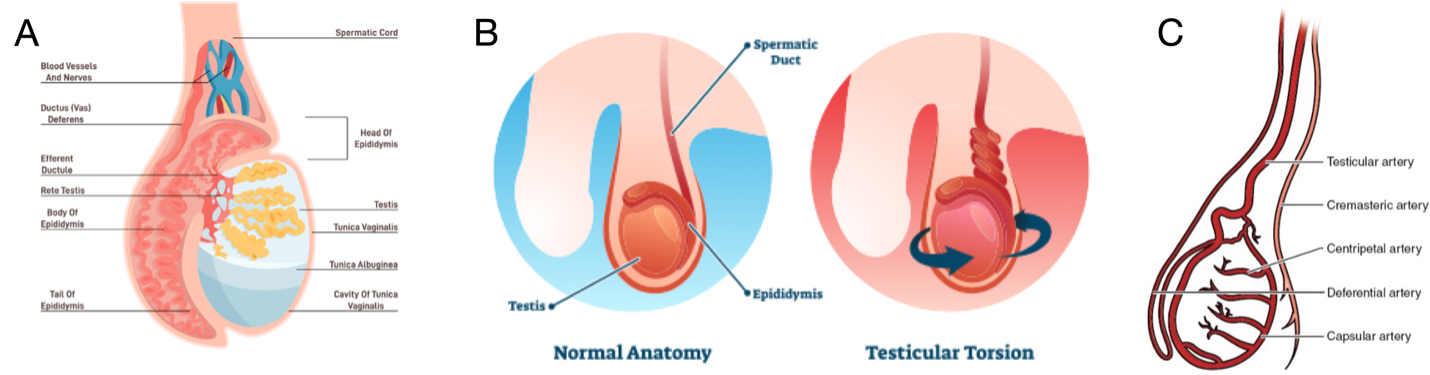
Testicular torsion (TT) is a common urological emergency with the majority of cases occurring in adolescence, making this an important presentation to pediatric emergency departments (EDs). TT accounts for 10-15% of pediatric acute scrotum (1). Testicular torsion typically presents with acute onset, unilateral testicular pain, and may be associated with nausea and vomiting. The differential diagnosis of TT is broad and includes more common diagnoses such as epididymo-orchitis and torsion of the appendix testis (1).
Cases that are highly suggestive of testicular torsion warrant immediate consultation with urology for de-torsion; however, in cases where the diagnosis is uncertain or at the request of the surgical team, doppler ultrasound is used to confirm the diagnosis prior to surgical intervention.
Certain clinical features are highly suggestive of the diagnosis of TT. The Testicular Workup for Ischemia and Suspected Torsion (TWIST) score (Table 1) was developed to help diagnose TT and decrease the use of ultrasound (2,3) . Low TWIST score (0-2) has a sensitivity 98% which allows ruling out TT and high TWIST (5-7) has specificity of 97% which allows ruling in the diagnosis. Ultrasound is helpful to confirm the diagnosis for the intermediate (2-4) risk group (2,4,5).
Table 1. TWIST score for clinical suspicion of testicular torsion.
| Testicular swelling |
2 points |
| Hard testis on palpation |
2 points |
| Absent cremasteric reflex |
1 point |
| High riding testis |
1 point |
| Nausea or vomiting |
1 point |
Delayed diagnosis of TT is associated with loss of testis and infertility. Cases that are highly suggestive of testicular torsion warrant immediate surgical consultation. The European Associatione of Urology 2024 recommendations for pediatric TT include that the clinical decision should be based on physical examination and ultrasound can be used as an adjunct that should not delay definitive care (6). In confirmed cases of TT, early surgical exploration is warranted.
Why PoCUS?
Studies consistently show that the most important factor for testicle viability is the duration of ischemia (7–9). Salvage of the testicle diminishes significantly after 6 hours, making early identification of this condition extremely important for improved patient outcomes (9). The median time from symptom onset to ED presentation for patients with possible TT is 4 hours – leaving only 2 hours for triage, ED physician assessment, work-up, urology consultation and transfer to the operating room to improve chances of testicular viability (7). Delays to definitive care in patients with TT are a preventable cause of orchiectomy in young men (7,10). PoCUS is an easily accessible bedside tool that can be used to expedite care for these patients. It can also help with resource allocation of our radiology performed ultrasounds, especially at centers where this is not readily available 24 hours a day. PoCUS can be used to rule in TT and has been shown to decrease time to OR for testicular torsion and decreased length of stay in ED (10,11).
Testicular PoCUS skills can be acquired rapidly. Competency and skill confidence was achieved by urology and emergency resident following a curriculum which included three audio lectures followed by 1-hour of hands-on practice (12). The test characteristics of PoCUS vs RADUS are demonstrated in Table 2.
Table 2: Test characteristics of radiology performed ultrasound vs. point-of-care ultrasound for testicular torsion (10)
| |
Radiology US |
Point of Care US |
| Sensitivity |
100% |
95% |
| Specificity |
98% |
93% |
| Positive predictive value |
83% |
46% |
| Negative predictive value |
100% |
100% |
| Median time for performing US |
61 minutes |
23 minutes |